
UNDERGRADUATE RESEARCH SYMPOSIUM


| UNDERGRADUATE RESEARCH SYMPOSIUM | 
|
|---|
Undergraduate Symposium: 5:00-6:00 P.M.
Social Hour: 6:00- 7:00 P.M.
Dinner: 7:00 P.M.
Meeting: 8:00 P.M.
Student: Lela Vukovic
Advisor: Prof. Cynthia J. Jameson
Department of Chemistry, University of Illinois at Chicago, Chicago, Illinois
ABSTRACT:
Many experiments utilize the interaction of Xe electrons with molecules
in its environment and verify
the large sensitivity of the Xe atom chemical shift arising from changes
in shielding of the Xe nucleus.
When in the presence of paramagnetic species, Xe exhibits particularly
large chemical shifts, arising from
the unpaired electrons. The chemical shift measured for a macroscopic sample
of xenon in oxygen arises
from three contributions: spin density at Xe nucleus, the usual intermolecular
contribution observed also
in diamagnetic systems, and contribution arising from the specific shape
of the sample on which the
measurement was made. Paramagnetic chemical shifts overwhelm other contributions,
and they arise
directly from the Xe hyperfine tensor. The hyperfine tensor consists of
the isotropic Fermi contact
interaction term and the traceless dipolar interaction term. In Xe-O2 gas
mixtures, only the Fermi contact
term of the hyperfine tensor contributes to the chemical shift. On the
other hand, the dipolar part provides
a strong relaxation mechanism for 129Xe. Using Xe as a probe to
investigate other more complicated
paramagnetic systems, particularly in solids, can become very useful, where
the hyperfine tensor can
provide information about distances (the Fermi term) and orientations (the
dipolar term) of paramagnetic
centers in the Xe environment.
In order to obtain the Xe hyperfine tensor, we carried out quantum mechanical calculations (B3LYP/DFT) for 70 different configurations of Xe with respect to O2 center of mass. The Fermi contact term varies for different configurations due to different densities of the unpaired spin at different positions in space. The nuclear shielding was calculated for the same configurations. We verify the accuracy of our quantum calculations by using the calculated values to predict the density and temperature dependence of Xe chemical shifts in oxygen-xenon gas mixtures that are infinitely dilute in Xe. Previous experiments on Xe-O2, in low density and low temperatures, show that the chemical shift of Xe in paramagnetic environment exhibits 1/T temperature dependence. When compared to Xe in diamagnetic gases (such as N2 or CH4), the shifts of Xe in Xe-O2 mixtures are unusually large in both the magnitude and temperature dependence.
This analysis is a fundamental step towards quantitatively understanding hyperfine shifts of Xe in the presence of paramagnetic centers in solids.
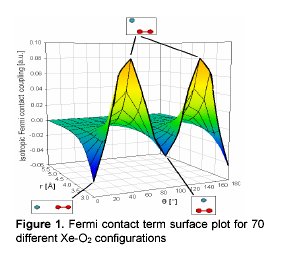 |
 |
1 C.J. Jameson, A.K. Jameson, and S.M. Cohen, Molec. Phys., 29,
1919 (1975)
2 C. J. Jameson, A. K. Jameson, et al.
Students: Abby Farning and Kathleen
Gongaware
Advisor: Jeffrey A. Jankowski
North Central College,
Naperville, IL 60540
ABSTRACT:
Since the late 1960’s, all Indy-type race cars have been
fueled by methanol. The primary reason for this switch was safety. Methanol
is water-soluble and can be extinguished with aqueous fire-fighting solutions – even
water. Ironically, the use of methanol presents an alternate fire-related
safety hazard. Methanol is such a clean-burning fuel, that the flames that
emanate from it are a faint blue - so faint, in fact, that in daylight conditions
they are effectively invisible. This creates a significant hazard to drivers,
pit crews, and firefighters involved in these races.
One of the goals of this research is to identify potential additives for methanol fuel by investigating the luminosity of flames from methanol/additive mixtures. Toward that end, investigations of the chemical basis of flame luminosity based on specific functional groups have been undertaken. A viable test method to quantitate the luminosity of flames produced from burning a sample of fuel has been established. With this method, recent investigations have been made on the effect of aromatic substiuent groups on flame luminosity when such compounds are blended with methanol. This has led to the examination of flame luminosity as a function of unsaturation and conjugation present in selected hydrocarbons when such compounds are used as additives in a methanol fuel mixture.